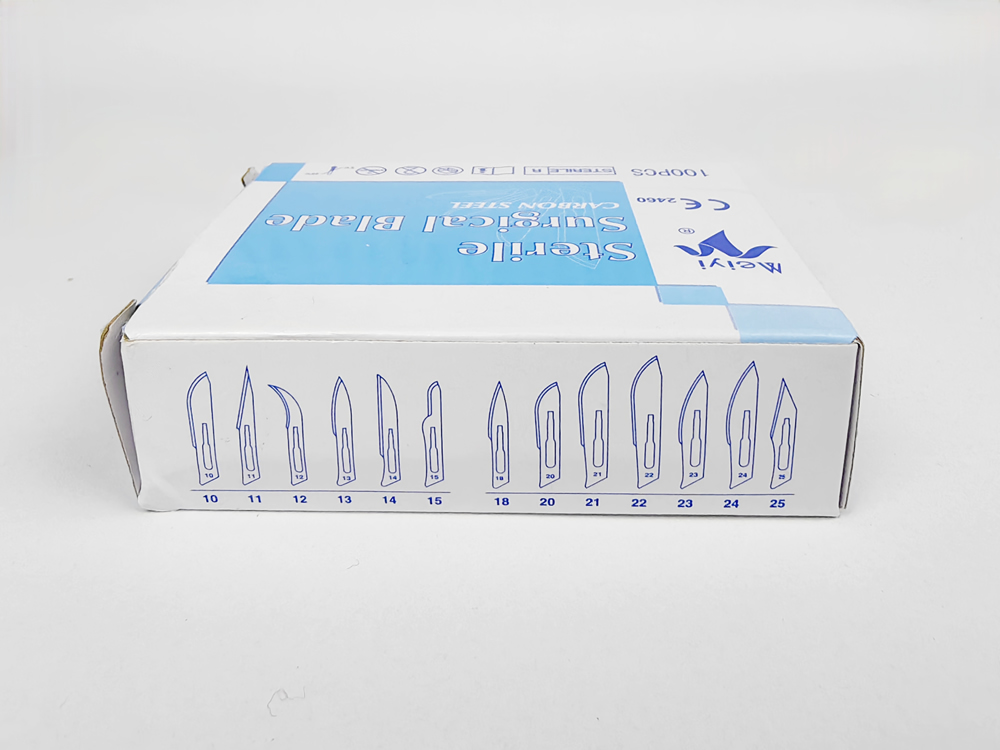
01
High Quality Medical EquipmentImplant Materials & Artificial Organs COX II
Screw dominance
Smaller connector volume, reduce the occupation for human tissue and irritability;The space of polyaxial screw in connecting rod is reduced, and the space of transvere connector and connecting rod is increased. Reduce the connector on the spinous process, transverse process interference, improve the flexibility of polyaxial screw, reduce the difficulty of surgery Bullet tip can make the implantation more easier;
The double thread thread make implantation speed more faster; Enhance the pedicle screw strength, with better pull-out resistance and bending resistance Barb thread design, minimize the expansion of screw connector, to prevent screw plug pull out, avoid thread wrong buckle; Deepened screw thread design. Optimize the polyaxial screw structure, the maximum assembly specification is φ 7.5.The pedicle screw structure has higher anti-spin ability (8N·m), and the ball head of thread part is not easy to come out; Press block release strength is up to 1600N, prevent press block release out.
description2
Attention and Suggestive Description
Please read the instruction before use carefully; Doctors should make clear the detailed notes to Patients in preoperative; If found have scratched the surface, broken, bent, crack phenomenon, the product is no longer available when delivered product; Implants and instruments provided by our company are non sterilization, but the surgical instruments must be sterilized by high-pressure steam sterilization process before using.



Indications
Various types of spinal fractures, including spinal burst fractures, compression fractures and fracture dislocation; short segment kyphosis orthopedic fixation; single segmental spinal instability or degenerative diseases.
Contraindications
Abnormal bone structure; nerve root canal anatomical abnormalities; serious neurological disorders; obesity; severe osteoporosis metal allergy.
FAQ
Q: What's your company Type? What certificates does Fule have?
A: We are manufacturer since 1994,with CE certificate, ISO13485:2003, ISO 9001:2008 and GMP,we can also work on it if you need some extra demands, OEM is always available.
Q: Is it possible to buy items different from your design ?
Answer: Yes, customized items are always welcome, while it will take longer time for delivery in this situation. We will try our best to help customers any way.
Question: How about the shippment?
A: Usually our shippment depends on the customers’ requirements by door to door courier service such as FEDEX, DHL, UPS and TNT, meantime,shipping by sea or air are available as well.
Q: Where is your factory located? How can I visit there?
A: Fule is located in Beijing, Pinggu District, which is very close to Beijing Airport. Welcome to visit Fule company.
description2